by Dr. Gesa Schulz
Isotopes are atoms of the same element with the same number of protons but different numbers of neutrons in the atomic nucleus. Therefore, isotopes differ in their atomic mass. They are divided into stable isotopes and unstable isotopes, with the latter exhibiting radioactive decay over time. The analysis of stable isotopes is a frequent used tool in the natural sciences, as it allows to determine sources and transformation processes. Even if the differences in the atomic masses are very small, they still lead to a different “reactivity” of the different isotopes of an element. The chemical bonds between so-called “heavy” isotopes, which have more protons than their “light” relatives, are stronger and therefore heavy isotopes react slower. An example: in microbiological turnover, microorganisms generally prefer the light isotopes and leave the heavy stronger bounded isotopes behind. This process is called “kinetic fractionation” and it enables us to use isotope analysis to investigate turnover processes and sources of elements.
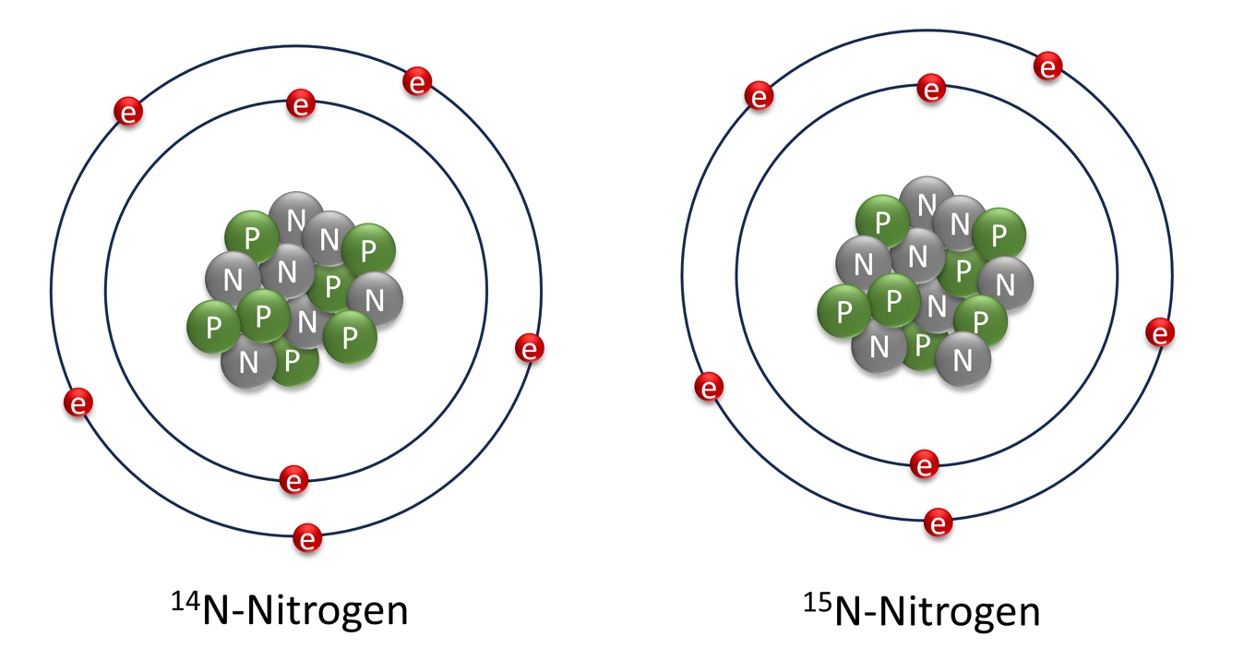
At SO305, we are studying the nitrogen cycle. Nitrogen is one of the most important elements for biological systems, as it is an essential nutrient for all organisms. Nitrogen exists in various forms, which can be reactive or non-reactive, and can be rapidly converted by microorganisms. However, most organisms can only process reactive nitrogen. If only limited amounts of reactive nitrogen are available, life in the ocean is “nitrogen-limited”. Nitrogen has two stable isotopes: the more abundant light 14N (99.635 %) and the rare heavy 15N (0.365 %).
In the Bay of Bengal, nitrogen is important as its turnover processes are sensitive to oxygen. A pronounced zone with low oxygen levels already exists here and we currently investigate how the increasing nitrogen inputs caused by anthropogenic impacts change the occurring processes. If oxygen levels fall below a critical threshold, nitrogen degradation can occur, in which microorganisms adapted to low oxygen concentrations convert reactive nitrogen to unreactive N2. Around 30-50 % of all reactive nitrogen loss currently takes place in oxygen minimum zones, although they only account for around 0.1 % of the ocean volume. In the Bay of Bengal, this critical threshold has not yet been reached, but the system may be approaching a tipping point due to increasing inputs and climate change.
As the working group “Biogeochemistry in the Earth System” of the University of Hamburg in cooperation with “Aquatic Nutrient Cycles” of the Helmholtz Center Hereon, we study the nitrogen cycle with the help of stable isotopes: we take samples for the natural stable isotopes in the water column and filter large quantities of water to determine the isotopes of nitrogen bound to the particles in the water. We cannot measure the isotopes on board, we take all our samples back to Germany. During the cruise, we are mainly filtering. Either small volumes via syringe filters to take the frozen filtrates back home, or large volumes for the filters on which we analyze the particulate nitrogen.
In addition to the filtration, we also use isotopes in incubation experiments in which we determine process rates. We do not measure which isotopes occur naturally, but instead label our samples with heavy 15N nitrogen. Over time, we track the turnover of the heavy isotope and use this to calculate the process rates.
At the end of the trip, we are faced with large quantities of frozen and cooled samples (approx. 80 liters of water and 200 filters as planned) that must be transported back to Germany to be measured for their isotopic composition in the lab.



